What are we studying? | Why are we studying? | What are we finding? | What does it mean? | Where can I read more?
What are we studying?
Hexavalent chromium (Cr(VI)) is known to cause lung cancer in humans. What is unknown is how it causes cancer. Lung cancer exhibits a type of genomic instability called chromosome instability, which is characterized by changes in both chromosome structure and number. We are the first lab to demonstrate that hexavalent chromium causes chromosome instability, DNA double strand breaks and neoplastic transformation in human lung cells. Now we are investigating how it causes these effects. We hope that by determining how hexavalent chromium causes genomic instability we might identify how to prevent chromium-induced cancers and events that are important to lung cancer progression in general that might lead to new treatment approaches or new ways to avoid lung cancer.
Why are we studying it?
Hexavalent chromium is a major environmental problem. Estimates now indicate that at least over a thousand hazardous waste sites on the National Priority List contain high levels of hexavalent chromium. At one site, soil levels of chromium have reached 19,000 mg/kg, which is extraordinarily high compared to normal levels (range of 0-250 mg/kg). Atmospheric levels of particulate Cr(VI) compounds can range from 1-100 ng/m3, which can lead to an average lung burden of 2 ug/day. Further environmental exposure comes from cigarette smoke; 1 cigarette can produce up to 0.5 ug of Cr. Superimposed on the amount of Cr that can come from any one exposure route is the fact that Cr accumulates in the body. Autopsies of Cr-exposed workers have shown that Cr accumulates and persists in the lungs for as long as 20 years after exposure. Thus, the general public is exposed to significant levels of Cr.
What are we finding?
We believe that a key event in how hexavalent chromium causes cancer is its ability to cause genomic instability. Below, we present and discuss our evidence that hexavalent chromium induces genomic instability which we see more specifically as chromosome instability with both numerical and structural changes in the chromosomes.
Overview of Hexavalent Chromium (Cr(VI))
We believe that a key event in how hexavalent chromium causes cancer is its ability to cause genomic instability. Below, we present and discuss our evidence that hexavalent chromium induces genomic instability which we see more specifically as chromosome instability with both numerical and structural changes in the chromosomes.
Chromosome Instability Studies
Numerical Chromosome Instability
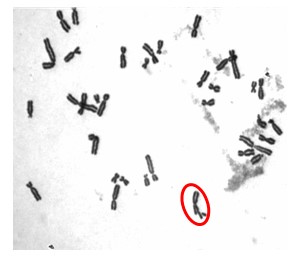
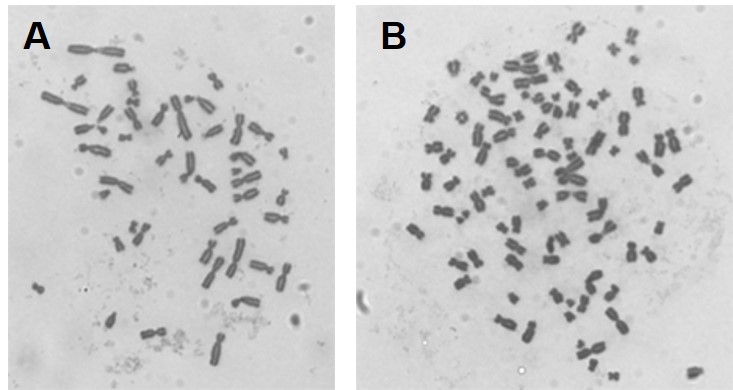
Our initial work determined that particulate chromate (shown in Figure 1) induced numerical chromosome instability. Figure 2A shows a normal metaphase with a normal number of chromosomes, 46. Figure 2B shows a metaphase with particulate chromate-induced chromosome instability (note that there are 92 chromosomes in the picture). Further study shows that there is an increase in both the number of metaphases with too few chromosomes (hypodiploid) and the number of metaphases with twice the number of chromosomes (tetraploid).
Chromosome Instability
Structural Chromosome Instability
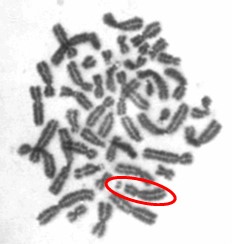
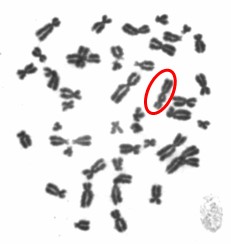
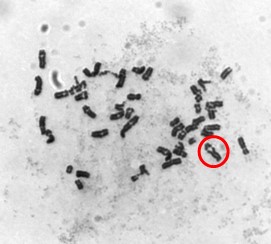
One type of chromosome instability that we are investigating is structural instability resulting from DNA damage after particulate chromate exposure (shown in Figure 1). Changes in chromosome structure can lead to deletions of genes or rearrangements that increase the levels of some proteins and decrease others. We found that particulate chromate can indeed damage chromosome structure causing chromatid lesions (Figure 2A), isochromatid lesions (Figure 2B), dicentric chromosomes (Figure 2C), and centromere spreading (Figure 2D).
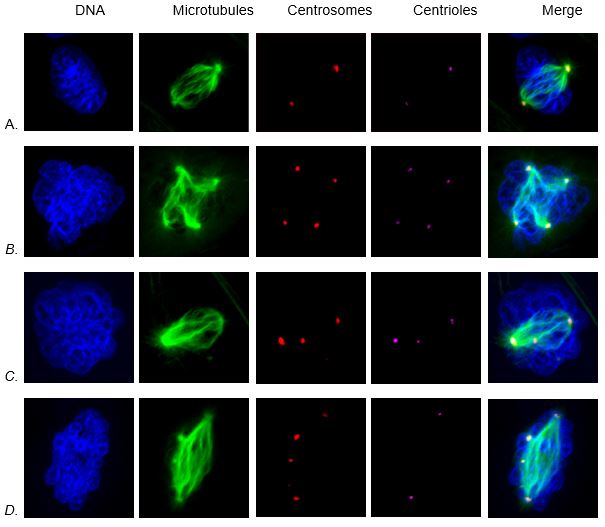
We next began exploring the potential cause of this effect. We started by considering the effects on centrosomes, the structures that direct cell division by controlling the mitotic spindle. Centrosome amplification (cells with greater than two centrosomes) is a hallmark of lung cancer and can induce numerical chromosome instability by unequally pulling the chromosomes into the daughter cells. Figure 3A shows a normal cell with the normal number of centrosomes (two). We found that particulate chromate induced centrosome amplification producing cells with four to eighteen centrosomes (Figures 3B-C).
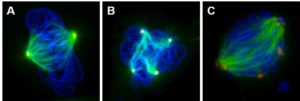
We also considered whether bypass of the spindle assembly checkpoint was playing a role. The spindle assembly checkpoint is a set of cellular proteins that operate as a network to regulate and monitor assembly of the mitotic spindle, which allows growing cells to divide into two cells. This checkpoint prevents cell division until all of the chromosomes are attached to the spindles and ready to separate. Bypassing the spindle assembly checkpoint can cause aneuploidy.
We found that particulate chromate does indeed cause spindle assembly checkpoint bypass. Normal cells with a properly functioning checkpoint should stop in metaphase when exposed to demecolcine. Cells treated with particulate chromate, however, bypass the checkpoint and continue through metaphase into anaphase which is seen as premature anaphase and premature centromere division. Figures 4A and 4B show cells treated with particulate chromate exhibiting premature anaphase (Fig. 4A) or premature centromere division (Fig. 4B).
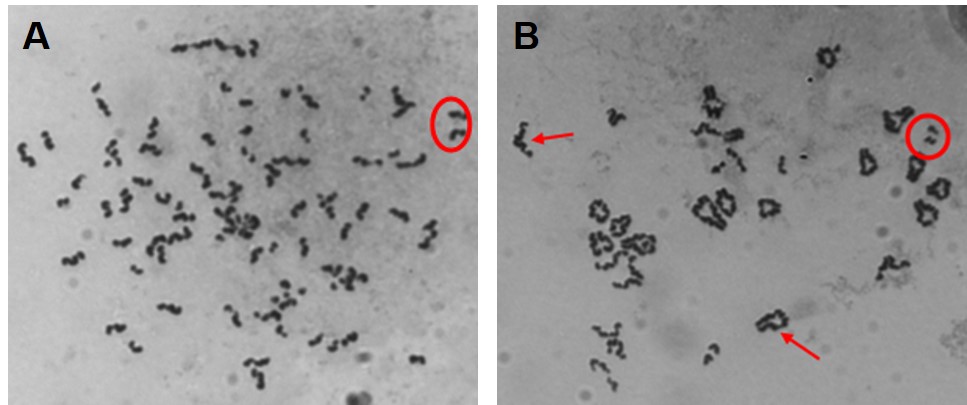
Structural Chromosome Instability
The other type of chromosome instability is structural and we are investigating that too. Changes in chromosome structure can lead to deletions of genes or rearrangements that increase the levels of some proteins and decrease others. We found that particulate chromate can indeed damage chromosome structure causing chromatid lesions (Figure 5A), isochromatid lesions (Figure 5B), dicentric chromosomes (Figure 5C), and centromere spreading (Figure 5D).
Currently, we are determining if particulate chromate can induce a particular type of structural damage called translocations using a technique called spectral karyotyping. This technique uses fluorescent dyes to paint each pair of chromosomes a different color (Figure 6A). Then by examining each chromosome, we can see when two chromosomes exchange pieces called a translocation. In Figure 6B, we show a translocation between chromosomes 2 and 5.
|
|
We have also begun investigating what causes these chromosome aberrations, focusing on DNA double strand breaks. DNA double strand breaks are a particularly dangerous type of DNA damage that may lead to chromosome damage.
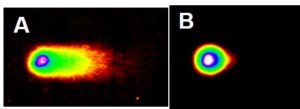
We were the first to discover that particulate chromate induced DNA double strand breaks. Figures 7 and 8 show two different measures of such damage. Figure 7A shows DNA fragmentation in a neutral gel. The picture resembles a comet with the tail of the comet consisting of DNA fragments caused by double strand breaks. Figure 7B shows control cell that has not been treated, and the picture therefore displays minimal tail and more closely resembles a moon.
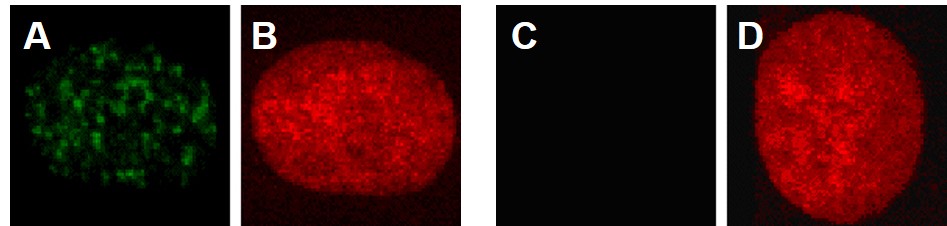
When double strand breaks form, gamma-H2A.X (g-H2A.X) rapidly accumulates on them and labels the break for repair. Use of a g-H2A.X-specific fluorescent antibody, will detect this protein at a double strand break site and appear as a glowing green dot. Each dot represents a double strand break in the DNA. Figure 8A shows the formation of these g-H2A.X foci in human lung cells treated with particulate chromate (note the many green dots). The cells in red are the same cells treated with a stain that only recognizes DNA. Figure 8B shows the absence of these dots, as there are no green dots, but the same cell is positive for the presence of DNA (red stain).
In response to particulate Cr(VI)-induced DNA double strand breaks, various proteins are activated in order to repair the damage. For example, after exposure to Cr(VI), ATM and SMC1 are phosphorylated and MRE11 expression is elevated (Figure 9). Mre11 functions as a damage sensor and sends the signal to the ATM kinase which then phosphorylates downstream proteins such as SMC1, to initiate cell cycle arrest allow time for the cell to repair the damage. When we knock down MRE11 expression by siRNA silencing, double strand break repair is reduced. Deficiencies in double strand break repair can lead to chromosome instability and cancer formation. To investigate the relationship between double strand break repair and carcinogenicity, we developed a model of neoplastic transformation based on human lung epithelial cells. Chronic exposure to particulate chromate leads to neoplastic transformation causing cell loss of contact inhibition and anchorage dependent in human lung epithelial cells (Figure 10).
DNA Repair
Homologous Recombination Repair
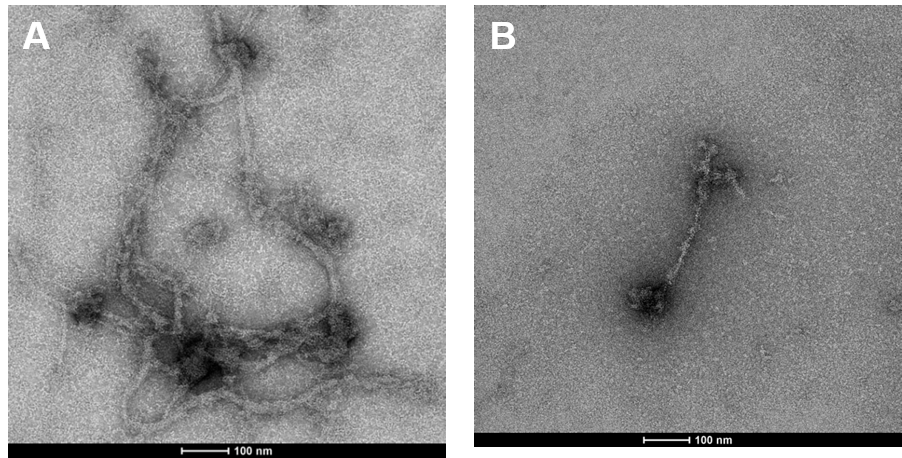
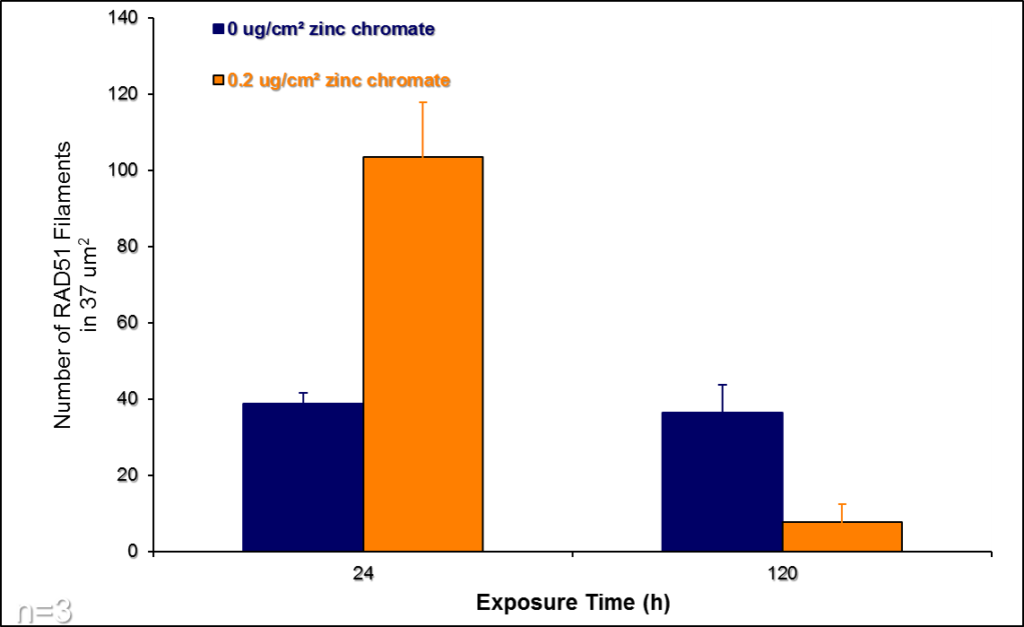
In response to particulate Cr(VI)-induced DNA double strand breaks, various proteins are activated in order to repair the damage. Rad51 is the signature protein for homologous recombination repair. It is the central component of a nucleoprotein filament that searches for the correct DNA sequence to carry out the necessary repair. We find the Cr(VI) inhibits the function of Rad51. Figure 11 show a Rad51 fiber. Figure 11A shows the complex fiber that forms in a normal cell that repair breaks after a 24 hour exposure to Cr(VI). Figure 11B shows how particulate Cr(VI) induces a much less complex fiber after 120 hours of exposure. Figure 12 shows that Cr(VI) initially increases the number of fibers after a 24 hour exposure as it increases repair of the breaks. However, Figure 12 shows that Cr(VI) inhibits the formation of these fibers and, thus the repair of these breaks with more prolonged exposures.
Current efforts are aimed at understanding how particulate chromate-induced DNA strand breaks, both single and double, are repaired and the genetic pathways involved in that repair. We are also trying to determine if double strands breaks are linked to numerical chromosome instability.
What does it mean?
Our data support the idea that Cr(VI) causes cancer by causing genomic instability. We find that while Cr(VI) causes damage and it is also inhibiting the repair of the damage making the damage even more extensive as it cannot be fixed. These concepts fundamentally alter the understanding of how Cr(VI) causes cancer.
Where can I read more?
- Wise, J.P., Leonard, J.C. and Patierno, S.R. Clastogenicity of Lead Chromate Particles in Hamster and Human Cells. Mutation Research, 278: 69-79, 1992. PMID: 1370121.
- Xu, J., Wise, J.P. and Patierno, S.R. DNA Damage Induced by Carcinogenic Lead Chromate Particles in Cultured Mammalian Cells. Mutation Research, 280: 129-136, 1992. PMID: 1378537.
- Wise, J.P., Orenstein, J.M. and Patierno, S.R. Inhibition of Lead Chromate Clastogenesis by Ascorbate: Relationship to Particle Dissolution and Uptake. Carcinogenesis, 14: 429-434, 1993. PMID: 8453719.
- Clawson, G. A., Norbeck, L.L., Wise, J.P. and Patierno, S.R. An Inhibitor of Nuclear Scaffold Protease Blocks Chemically Induced Transformation of Embryonic Fibroblasts. Cell Growth and Differentiation, 4: 589-594, 1993. PMID: 8398899.
- Wise, Sr., J.P., Stearns, D.M., Wetterhahn, K.E. and Patierno, S.R. Cell-Enhanced Dissolution of Carcinogenic Lead Chromate Particles: The Role of Individual Dissolution Products in Clastogenesis. Carcinogenesis, 15: 2249-2254, 1994. PMID: 7955062.
- Manning, F.C.R., Blankenship, L., Wise, J.P., Xu, J., Bridgewater, L.C. and Patierno, S.R. Induction of Internucleosomal Fragmentation by Carcinogenic Chromate: Relationship to Genotoxicity, Cell Cycle Delay and Inhibition of Macromolecular Synthesis. Environmental Health Perspectives, 102: 159-167, 1994. PMID: 7843091.PMCID: PMC1567430.
- Patierno, S.R., Blankenship, L.J., Wise, J.P., Xu, J., Bridgewater, L.C. and Manning, F.C.R. Genotoxin-Induced Apoptosis: Implications for Carcinogenesis. In: Hu, V.W. (Ed), The Cell Cycle: Regulators, Targets and Clinical Applications. Plenum Press, New York, pp. 331-340, 1994. .
- Stearns, D.M., Wise, Sr., J.P., Patierno, S.R. and Wetterhahn, K.E. Chromium (III) Picolinate Produces Chromosome Damage in Chinese Hamster Ovary Cells. The FASEB Journal, 9: 1643-1648, 1995. PMID: 8529845.
- Blankenship, J.J., Carlisle, D., Wise, Sr., J.P., Orenstein, J.M., Dye, L.E. and Patierno, S.R. Induction of Apoptotic Cell Death by Particulate Lead Chromate: Differential Effects of Vitamins C and E on Genotoxicity and Survival. Toxicology and Applied Pharmacology, 146: 270-280, 1997. PMID: 9344895.
- Wise, Sr., J.P. and Stearns, D.M. Chromium carcinogenicity: The Roles of Valence State and Solubility. The Occupational and Environmental Medicine Report (The OEM Report), 11(11): 100-103, 1997. PMID: Not applicable.
- Wise, Sr., J.P., Wise, S.S. and Little, J.E. The Cytotoxicity and Genotoxicity of Particulate and Soluble Hexavalent Chromium in Human Lung Cells. Mutation Research, 517: 221-229, 2002. PMID: 12034323.
- Wise, S.S., Elmore, L.W., Holt, S.E., Little, J.E., Antonucci, P.G., Bryant, B.H. and Wise, Sr., J.P. Telomerase-Mediated Lifespan Extension of Human Bronchial Cells Does Not Affect Hexavalent Chromium-Induced Cytotoxicity or Genotoxicity. Molecular and Cellular Biochemistry, 255: 103-111, 2004. PMID: 14971651.
- Wise, S.S., Schuler, J.H.C., Katsifis, S.P. and Wise, Sr., J.P. Barium Chromate Is Cytotoxic and Genotoxic to Human Lung Cells. Environmental and Molecular Mutagenesis, 42: 274-278, 2004. PMID: 14673872.
- Wise, S.S., Holmes, A.L., Ketterer, M.E., Hartsock, W.J., Fomchenko, E., Katsifis, S.P., Thompson, W.D. and Wise, Sr., J.P. Chromium Is the Proximate Clastogenic Species for Lead Chromate-Induced Clastogenicity in Human Bronchial Cells. Mutation Research, 560: 79-89, 2004. PMID: 15099827.
- Wise, S.S., Schuler, J.H.C., Holmes, A.L., Katsifis, S.P., Ketterer, M.E., Hartsock, W.J., Zheng T. and Wise, Sr., J.P. A Comparison of Two Carcinogenic Particulate Hexavalent Chromium Compounds: Barium Chromate Is More Genotoxic than Lead Chromate in Human Lung Cells. Environmental and Molecular Mutagenesis, 44: 156-162, 2004. PMID: 15278919.
- Xie, H., Holmes, A.L., Wise, S.S., Gordon, N. and Wise, Sr., J.P. Lead Chromate-Induced Chromosome Damage Requires Extracellular Dissolution to Liberate Chromium Ions but Does Not Require Particle Internalization or Intracellular Dissolution. Chemical Research in Toxicology, 17(10): 1362-1367, 2004. PMID: 15487897.
- Holmes, A.L., Wise, S.S., Xie, H., Gordon, N., Thompson W.D. and Wise, Sr., J.P. Lead Ions Do Not Cause Human Lung Cells to Escape Chromate-Induced Cytotoxicity. Toxicology and Applied Pharmacology, 203: 167-176, 2005. PMID: 15710177.
- Xie, H., Wise, S.S., Holmes, A.L., Xu, B., Wakeman, T., Pelsue, S.C., Singh, N.P. and Wise, Sr., J.P. Carcinogenic Lead Chromate Induces DNA Double-Strand Breaks in Human Lung Cells. Mutation Research, 586(2): 160-172, 2005. PMID: 16112599. PMCID: PMC4136752.
- Wise, S.S., Holmes, A.L., Moreland, J.A., Xie, H., Sandwick, S.J., Stackpole, M.M., Fomchenko, E., Teufack, S., May, Jr., A.J., Katsifis, S.P. and Wise, Sr., J.P. Human Lung Cell Growth Is Not Stimulated by Lead Ions after Lead Chromate-Induced Genotoxicity. Molecular and Cellular Biochemistry, 279(1-2): 75-84, 2005. PMID: 16283516.
- Grlickova-Duzevik E., Wise, S.S., Munroe, R.C., Thompson, W.D. and Wise, J.P., Sr. XRCC1 Protects against Particulate Chromate-Induced Chromosome Damage and Cytotoxicity in Chinese Hamster Ovary Cells. Toxicological Sciences, 92(1): 96-102, 2006. PMID: 16597656.
- Holmes, A.L., Wise, S.S., Sandwick, S.J., Lingle, W.L., Negron, V.C., Thompson W.D. and Wise, Sr., J.P. Chronic Exposure to Lead Chromate Causes Centrosome Abnormalities and Aneuploidy in Human Lung Cells. Cancer Research, 66(8): 4041-4048, 2006. PMID: 16618723.
- Duzevik, E.G., Wise, S.S., Munroe, R.C., Thompson, W.D. and Wise, Sr., J.P. XRCC1 Protects against Particulate Chromate-Induced Chromosome Damage and Cytotoxicity in Chinese Hamster Ovary Cells. Toxicological Sciences, 92(2): 409-415, 2006. PMID: 16714390.
- Wise, S.S., Holmes, A.L. and Wise, Sr., J.P. Particulate and Soluble Hexavalent Chromium Are Cytotoxic and Genotoxic to Human Lung Epithelial Cells. Mutation Research, 610(1-2): 2-7, 2006. PMID: 16872863.
- Holmes, A.L., Wise, S.S., Sandwick, S.J. and Wise, Sr., J.P. The Clastogenic Effects of Chronic Exposure to Particulate and Soluble Cr(VI) in Human Lung Cells. Mutation Research, 610(1-2): 8-13, 2006. PMID: 16870495.
- Grlickova-Duzevik, E., Wise, S.S., Munroe, R.C., Thompson, W.D. and Wise, Sr., J.P. XRCC1 Protects Cells from Chromate-Induced Chromosome Damage, but Does Not Affect Cytotoxicity. Mutation Research, 610(1-2): 31-37, 2006. PMID: 16904935.
- Wise, S.S., Holmes, A.L., Xie, H., Thompson, W.D. and Wise, Sr., J.P. Chronic Exposure to Particulate Chromate Induces Spindle Assembly Checkpoint Bypass in Human Lung Cells. Chemical Research in Toxicology, 19(11):1492-1498, 2006. PMID: 17112237.
- Wise, Sr., J.P. Genotoxic and Carcinogenic Effects of Lead. In, S. EPA. Air Quality Criteria for Lead (Final). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-05/144aF-bF, 5-125 – 5-147, 2006.
- Savery, L.C., Grlickova-Duzevik, E., Wise, S.S., Thompson, W.D., Hinz, J.M., Thompson, L.H. and Wise, Sr., J.P. Role of the Fancg Gene in Protecting Cells from Particulate Chromate-Induced Chromosome Instability. Mutation Research, 626(1-2): 120-127, 2007. PMID: 17097336.
- Camrye, E., Wise, S.S., Milligan, P., Gordon, N., Goodale, B., Stackpole, M., Patzlaff, N., Aboueissa, A. and Wise, Sr., J.P. Ku80 Deficiency Does Not Affect Particulate Chromate-Induced Chromosome Damage and Cytotoxicity in Chinese Hamster Ovary Cells. Toxicological Sciences, 97(2): 348-54, 2007. PMID: 17361020.
- Stackpole, M.M., Wise, S.S., Goodale, B.C. Duzevik, E.G., Munroe, R.C., Thompson, W.D., Thacker, J., Thompson, L.H., Hinz, J.M. and Wise, Sr., J.P. Homologous Recombination Protects Against Particulate Chromate-Induced Genomic Instability in Chinese Hamster Cells, Mutation Research, 625: 145-154, 2007. PMID: 17662313; PubMed Central PMCID: PMC2230547
- Xie, H., Holmes, A.L., Wise, S.S., Huang, S., Peng, C. and Wise, Sr., J.P. Neoplastic Transformation of Human Bronchial Cells by Lead Chromate Particles, American Journal of Respiratory Cell and Molecular Biology, 37(5): 544-552, 2007. PMID: 17585 PMCID: PMC2048681.
- Xie, H., Wise, S.S. and Wise, Sr., J.P. Deficient Repair of Particulate Chromate-Induced DNA Double Strand Breaks Leads To Neoplastic Transformation. Mutation Research, 649: 230-238, 2008. PMID: 18023605. PMCID: PMC2230545.
- Brooks, B., O’Brien, T.J., Ceryak, S., Wise, Sr., J.P., Wise, S.S., Wise, Jr., J.P., DeFabo, E. and Patierno, S.R. Excision Repair is Required for Genotoxin-Induced Mutagenesis in Mammalian Cells. Carcinogenesis, 29(5): 1064–1069, 2008. PMID: 18332048. PMCID: PMC2902384.
- Wise, S.S., Holmes, A.L. and Wise, Sr., J.P. Hexavalent chromium-induced DNA damage and repair mechanisms. Reviews on Environmental Health, 23(1): 39-57, 2008. PMID: 18557597.
- Holmes, A.L., Wise, S.S. and Wise, Sr., J.P. Carcinogenicity of hexavalent chromium. Indian Journal of Medical Research, 128: 353-372, 2008. PMID: 19106434.
- Xie, H., Holmes, A.L., Young, J.L., Qin, Q., Joyce, K, Pelsue, S.C., Peng, C., Wise, S.S., Jeevarajan, A., Wallace, W.T., Hammond, D. and Wise, Sr., J.P. Zinc Chromate Induces Chromosome Instability and DNA Double Strand Breaks in Human Lung Cells. Toxicology and Applied Pharmacology, 234: 293–299, 2009. PMID: 19027772; PubMed Central PMCID: PMC4075174.
- Wise, J.P. Hexavalent Chromium Compounds. In: Schwab M. (Ed.). Encyclopedia of Cancer, 2nd ed. pp. 1374-1387. Springer, Berlin Heidelberg New York. 2009.
- Wise, S.S., Holmes, A.L., Qin, Q., Xie, H., Katsifis, S., Thompson, W.D. and Wise, Sr., J.P. Comparative Genotoxicity and Cytotoxicity of Four Hexavalent Chromium Compounds in Human Bronchial Cells. Chemical Research in Toxicology, 23(2): 365-372, 2010. PMID: 19632355. PMCID: PMC4048704.
- Holmes, A.L., Wise, S.S., Pelsue, S.C., Aboueissa, A., Lingle, W., Salisbury, J., Gallagher, J. and Wise, Sr., J.P. Chronic Exposure to Zinc Chromate Induces Centrosome Amplification and Spindle Assembly Checkpoint Bypass in Human Lung Fibroblasts. Chemical Research in Toxicology, 23(2): 386-395, 2010. PMID: 20030412. PMCID: PMC2822114.
- Wise Jr., J.P., Wise, S.S., Wise, J., McKay, J., Browne, M., Joyce, K., Braun, M., Wise, C.F., Duffy, R., Estell, E., Brown, J., Gianios Jr., C., Mason, M., Shehata, T., Hammond, D., Thompson, W.D. and Wise, Sr., J.P. Altered Gravity Exacerbates Chromate-Induced Genotoxicity. Gravitational and Space Biology, 23(2): 83-84, 2010. PMID: Not applicable..
- Holmes, A.L. and Wise, Sr., J.P. Mechanisms of Metal-Induced Centrosome Amplification. Biochemical Society Transactions, 38(6): 1687-1690, 2010. PMID: 21118148. PMCID: PMC4086844.
- Wise, S.S. and Wise, Sr., J.P. Aneuploidy as an Early Mechanistic Event in Metal Carcinogenesis. Biochemical Society Transactions, 38(6): 1650-1654, 2010. PMID: 21118142. PMCID: PMC4086856.
- Ali, A., Ma, Y., Reynolds, J., Wise, Sr., J.P., Inzucchi, S.E. and Katz, D.L. Chromium Effects on Glucose Tolerance, Insulin Sensitivity and Vascular Function in People at Risk for Diabetes. Endocrine Practice, 17(1):16-25, 2011. PMID: 20634174. PMCID: PMC3118091.
- Wise, J.P. Hexavalent Chromium Compounds. In: Schwab M. (Ed.). Encyclopedia of Cancer, 3rd ed. Springer, Berlin Heidelberg New York. 2012.
- Wise, S.S. and Wise, Sr., J.P. Chromium and Genomic Stability. Mutation Research, 733: 78– 82, 2012. PMID: 22192535. PMCID: PMC4138963.
- Kost, G.C., Patierno, S.R., Wise, S.S., Holmes, A.L., Wise, Sr., J.P. and Ceryak, S. Protein tyrosine phosphatase (PTP) inhibition enhances chromosomal stability after genotoxic stress: Decreased CIN at the expense of enhanced GIN? Mutation Research, 735: 51– 55, 2012. PMID: 22583656. PMCID: PMC3389208.
- Holmes, A.L. and Wise, Sr., J.P. Aneuploidy: Mechanisms, Cancer and the Role of Environmental Pollutants. Chapter 1, pp. 1-33 in de Rossi, S. and Bianchi, F. (eds.) Aneuploidy: Etiology, Disorders and Risk Factors. Nova Science Publishers Inc., Hauppauge, NY. 2012.
- Karri, N.D., Xie, H. and Wise, Sr., J.P. Chronic Exposure to Particulate Hexavalent Chromium Alters Cdc20 Protein Localization, Interactions and Expression. Journal of Carcinogenesis & Mutagenesis, 4(2): 140-147, 2013. http://dx.doi.org/10.4172/2157-2518.1000140.
- Wise, Sr., J.P. and Qin, Q. Hexavalent Chromium and DNA, Biological Implications of Interaction. In: Kretsinger, R.H., Uversky, V.N. and Permyakov, E.A. (Eds.). Encyclopedia of Metalloproteins. 1st ed. Springer, New York. Pages 975-977.2013. DOI 10.1007/978-1-4614-1533-6. ISBN 978-1-4614-1532-9
- Qin, Q., Xie, H., Wise, S.S., Browning, C.L., Thompson, K.N., Holmes, A.L., and Wise, Sr., J.P. Homologous Recombination Repair Signaling in Chemical Carcinogenesis: Prolonged Particulate Hexavalent Chromium Exposure Suppresses the Rad51 Response in Human Lung Cells. Toxicological Sciences, 142(1): 117–125, 2014. PMID: 25173789. PMCID: PMC4271064.
- Xie, H., Holmes, A.H., Wise, S.S., W. Young, J.L., Wise, J.T.F. and Wise, Sr., J.P. Human Skin Cells Are More Sensitive than Human Lung Cells to the Cytotoxic And Cell Cycle Arresting Impacts of Particulate and Soluble Hexavalent Chromium. Biological Trace Element Research, 166(1): 49-56, 2015. PMID: 25805272. PMCID: PMC4470775.
- Martino, J., Holmes, A.L., Xie, H., Wise, S.S., and Wise, Sr., J.P. Chronic Exposure to Particulate Chromate Induces Premature Centrosome Separation and Centriole Disengagement in Human Lung Cells. Toxicological Sciences 147(2): 490-499, 2015. PMID: 26293554. PMCID: PMC4635651.
- Xia, W., Hu, J., Zhang, B., Li, Y., Wise, Sr., J.P., Bassig, B.A., Zhou, A., Savitz, D.A., Xiong, C., Zhao, J., du, X., Zhou, Y., Pan, X., Yang, J., Wu, C., Jiang, M., Peng, Y., Qian, Z., Zheng, T., and Xu, S. A Case-Control Study of Maternal Exposure to Chromium and Infant Low Birth Weight in China. Chemosphere 144: 1484-1489, 2016. PMID: 26869268. PMCID: PMC4751650.
- Wise, S.S., Holmes, A.L., Liou, L., Adam, R.M., and Wise, Sr., J.P. Hexavalent Chromium Induces Chromosome Instability in Human Urothelial Cells. Toxicology and Applied Pharmacology, 296:54-60, 2016. PMID: 26908176. PMCID: Pending.
- Browning, C.L., Qin, Q., Kelly, D.F., Prakash, R., Jasin, M., and Wise, Sr., J.P. Prolonged Particulate Hexavalent Chromium Exposure Suppresses Homologous Recombination Repair in Human Lung Cells. Toxicological Sciences 153(1):70-78, 2016. PMID: 27449664. PMCID: Pending.
- Browning, C.L., Speer, R.M., and Wise, Sr., J.P. Molecular Mechanisms of Chromium-Induced Carcinogenesis in Mudipalli, A., and Zelikoff, J. (Eds) Essential and Non- Essential Metals: Carcinogenesis, Prevention and Therapeutics, Springer, UK, In press.
- Wise, S.S., and Wise, Sr., J.P. Metal Carcinogenesis and DNA damage: A Case Study using Hexavalent Chromium, Chapter 7 in Poirier, M.C. (Ed) Carcinogens, DNA Damage and Cancer Risk, World Scientific Publishers, Singapore, In press.