What are we studying? | Why are we studying? | What are we finding? | What does it mean? | Where can I read more?
What are we studying?
We are studying depleted uranium (DU) and its potential to cause cancer. DU-induced lung cancers occur in human bronchial cells. However, despite these observations, the effects of DU in human bronchial cells are poorly understood. Our work is focused on determining the effects of DU on DNA and genomic stability, and its potential to cause cancer. We are using human lung cells, to considering the ability of DU to damage DNA (measured as strand breaks and chromosome damage), affect DNA repair, induce genomic instability and transform normal cells into tumor cells.
Why are we studying it?
DU is becoming a wider international concern as a possible health hazard and carcinogen. Little is currently known about DU mechanisms of effect, but reported data have shown lung cancer, embryotoxicity and teratogenicity, reproductive and developmental damage, genomic instability and DNA damage in vitro. Given the widespread use of uranium for military applications and the present world-wide deployment of the United States military, it is important to better define both the risks of DU exposure and the possible mechanisms of carcinogenicity and genotoxicity.
What are we finding?
Cytotoxicity
We found both particulate and soluble DU compounds induced concentration-dependent increases in cytotoxicity in human lung cells.
Chromosome Instability
We assessed the clastogenicity of particulate and soluble DU compounds in human lung fibroblasts after a 24 h exposure and found concentrations of 0, 0.5, 1, and 5 ug/cm2 particulate uranyl trioxide (UO3), induced chromosome damage in 5, 6, 10, and 15% of metaphase cells. Soluble uranyl acetate did not induce chromosome damage above background levels. With longer exposures of 48 and 72 h there were slight increases in chromosome damage induced with UO3 treatment, but no meaningful increase in chromosome damage was observed with uranyl acetate.
In addition to bronchial fibroblasts, we have examined the cytotoxic and clastogenic effects of UO3 on human bronchial epithelial cells. We found a concentration-dependent increase in cytotoxicity, but a significant increase in chromosome damage is not seen until a 48 h exposure, suggesting a mechanism of toxicity which requires longer exposures to break DNA.
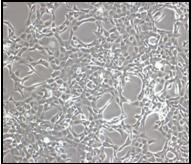
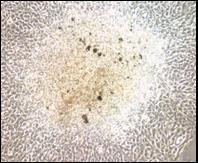
We used immortalized human bronchial epithelial cell lines to study the transforming potential of particulate DU. We observed focus formation in cells exposed to UO3 after a 24 h exposure shown in the figure below. Specifically, after 24 h UO3 treatment with 0, 0.25, 2.5, and 25 ug/cm2 UO3 we observed foci frequencies of 3, 23, 24, and 21 foci, respectively.
The DU transformants also acquired anchorage-independent growth. The figure below shows colonies in soft agar formed by DU-transformed cells. After 24 h treatment with concentrations of 0, 0.25, 2.5, and 25 ug/cm2 UO3, the percentage of resultant foci that grew colonies in soft agar was 0, 67, 100, and 75, respectively. In addition, 53% of DU-transformed cell lines tested showed a hypodiploid phenotype (chromosome number less than 44) with a significant increase in metaphases exhibiting a chromosome number ranging from 7 to 43.
 |
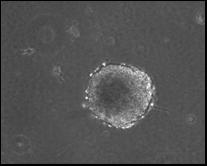 |
| DU-transformed cells formed colonies in soft agar medium. | |
We also considered the role of DNA damage and repair as a key factor in uranium’s carcinogenicity. We investigated how DU causes DNA strand breaks and chromosome aberrations. In particular, we looked at the effects of homologous recombination repair on DU-induced genotoxicity using RAD51D and XRCC3-deficient Chinese hamster ovary (CHO) cell lines; these proteins are important players in the HR pathway. We found that loss of these genes did not affect cell survival. However, DU induces more chromosome breaks and fusions in the XRCC3 deficient cells but loss of RAD51D had no increase in breaks. We also found that DU induced chromosome specific chromosome fragmentation of the X chromosome in wild type and RAD51D deficient cells but not in the XRCC3 deficient cells..
What does it mean?
Our data support the possibility that DU may poses a potential health risk to people who are exposed to it through either inhalation or other routes such as diet or drinking water. Furthermore, because our data indicate that particulate DU damages chromosomes and transforms human lung cells, DU exposure may contribute to the development of cancer.
Where can I read more?
- Wise, S.S., Thompson, W.D., Aboueissa, A., Mason, M.D. and Wise, Sr., J.P. Particulate Depleted Uranium Is Cytotoxic and Clastogenic to Human Lung Cells. Chemical Research in Toxicology, 20(5): 815-820, 2007. PMID: 17432880. PMCID: Not applicable.
- Xie, H., LaCerte, C., Thompson, W.D. and Wise, Sr., J.P. Depleted Uranium Induces Neoplastic Transformation in Human Lung Epithelial Cells. Chemical Research in Toxicology, 23(2): 373-378, 2010. PMID: 20000475.
- LaCerte, C., Xie, H., Aboueissa, A. and Wise, Sr., J.P. Particulate Depleted Uranium Is Cytotoxic and Clastogenic to Human Lung Epithelial Cells. Mutation Research, 697: 33–37, 2010. PMID: 20172046.
- Holmes, A.L., Joyce, K., Falank, C., Xie, H. Hinz, J. and Wise, Sr., J.P. The Impact of Homologous Recombination Repair Deficiency on Depleted Uranium Clastogenicity in Chinese Hamster Ovary Cells: XRCC3 Protects Cells from Chromosome Aberrations, but Increases Chromosome Fragmentation. Mutation Research, 762: 1-9, 2014. PMID: 24561002.